- Home
- Pricing
- About Us
- Partners
- Jobs
- Contact Us
- All in One Data Platform
- Fully Customizable
- Adaptable Widgets
- Process Integration
- AI Integration
- Hardware & Software Integration
- Top Tier Security
- User Friendly Interface
- Professional & Speedy Support
- Data Centers
- Barcode/QR Code System
- articleElectronic Lab Notebook (ELN)
- inventory_2Inventory Management
- scienceLaboratory Information Management Systems (LIMS)
- calculateFormulation Management
- yardAgricultural Research Manager (ARM)
- health_and_safetyQuality Management System (QMS)
- groupsCustomer Relationship Management (CRM)
- todayProject Management
- shopping_cartPurchase Management
- descriptionReference Management
- differenceOther Applications
- Biotech and Pharmaceutical Companies
- Contract Research Organizations (CROs)
- ELN/LIMS Providers
- Any Organization Needing Data Documentation
- Login
- Free signup
- Request a demo
- Referral Program
- Subscribe to our newsletter
- Documentation
- Blogs
- Resources
- Screenshots
- Downloads
- Terms of Service
- Privacy Policy
Risk Assessment
Overview
Labii Risk Assessment empowers research, manufacturing, and compliance teams to identify, evaluate, and mitigate risks with precision. Built for life sciences, pharmaceuticals, and other regulated industries, Labii makes risk management a structured, auditable, and repeatable process—no spreadsheets required.
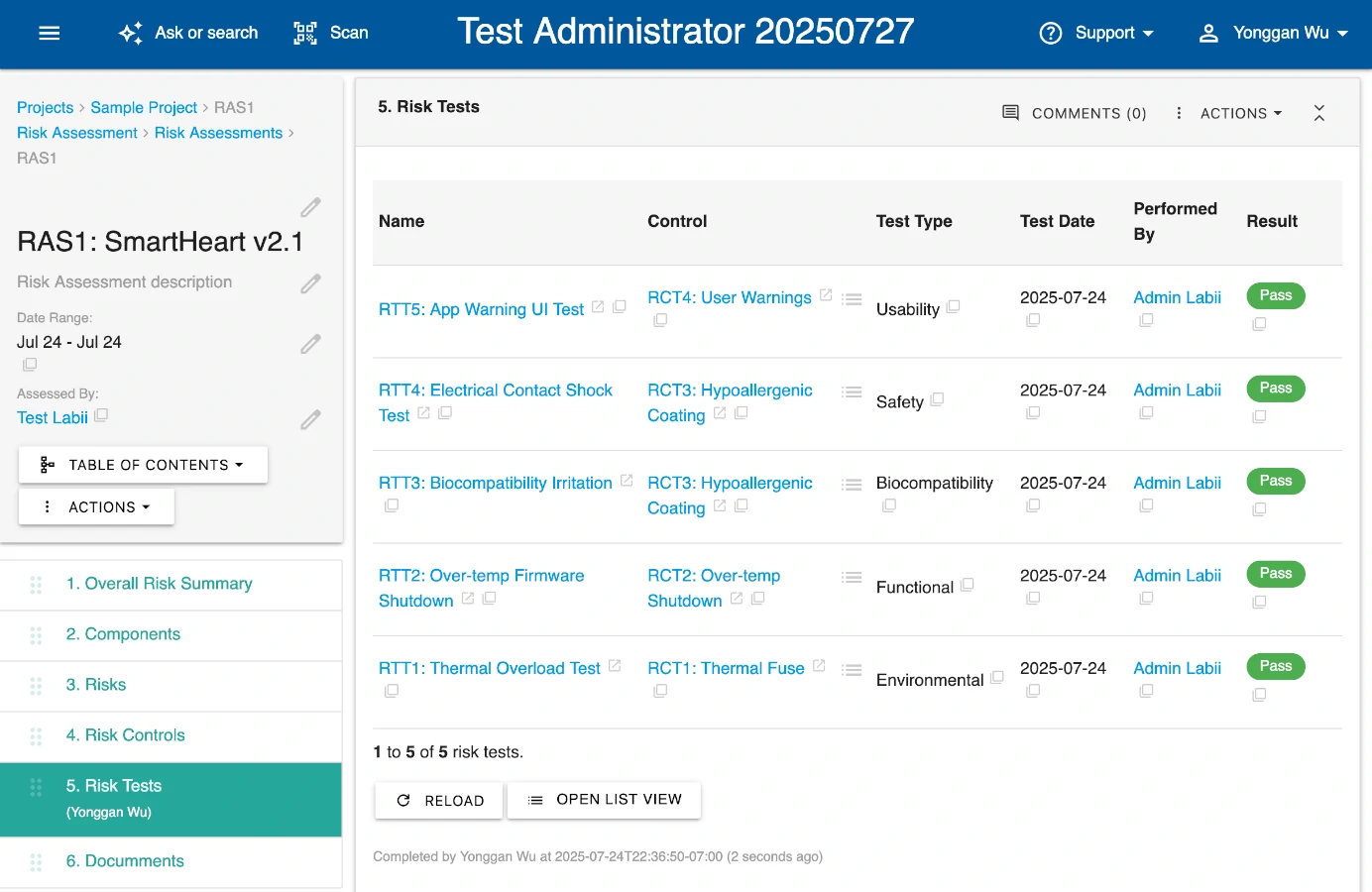
Centralize Risk Documentation and Control
Labii provides a unified workspace to manage risk registers, hazard analyses, and mitigation strategies. Whether you’re implementing ISO 14971, HACCP, or ICH Q9, Labii adapts to your regulatory needs while maintaining full traceability.
Customizable Risk Templates for Any Workflow

Choose from pre-built templates or create your own risk models with configurable matrices, thresholds, categories, and evaluation rules. Labii’s modular system aligns with your SOPs and quality system requirements.
Quantitative and Qualitative Risk Analysis
Score risks by severity, likelihood, and detectability. Labii automatically calculates Risk Priority Numbers (RPNs) and presents them in intuitive dashboards and heatmaps, making prioritization and follow-up simple.

Built-In Structure: Data Tables for Full Traceability
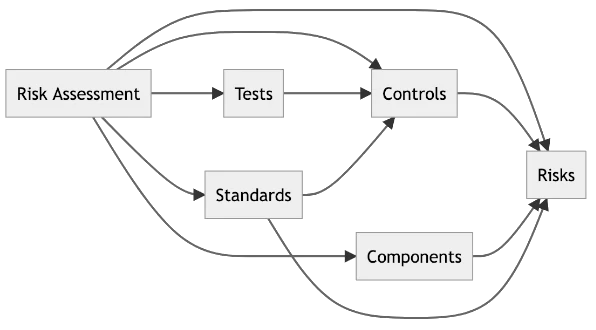
Labii’s Risk Assessment module is engineered with a flexible, normalized table structure to support granular data tracking, modular assessments, and scalable evaluations. This design allows Labii to serve as both a risk register and a risk control evidence library—ideal for compliance, traceability, and impact analysis.
- Risk Assessment – The master record that ties together a complete risk review or evaluation. Can represent a device, process, system, or product under assessment.
- Risk – Individual risk items identified and assessed within a given risk assessment. Includes severity, probability, and detectability scores.
- Risk Control – Captures preventive or corrective actions taken to reduce risk, including their effectiveness, type (e.g., design, procedural), and residual risk outcomes.
- Risk Standard – A reference table of regulatory or internal standards used as guidance (e.g., ISO 14971, ICH Q9), ensuring alignment with best practices.
- Risk Component – Breaks down assessments by sub-systems, components, or steps in a process—supporting modularity and reuse across assessments.
- Risk Test – Records any verification or validation activities used to confirm the effectiveness of risk controls, ensuring documented evidence for audits.
Collaborate Securely Across Teams
Enable cross-functional teams to work together on risk assessments. Labii offers role-based access control, threaded comments, and full version history for traceable decision-making.
Seamless Integration with ELN, LIMS, and QMS
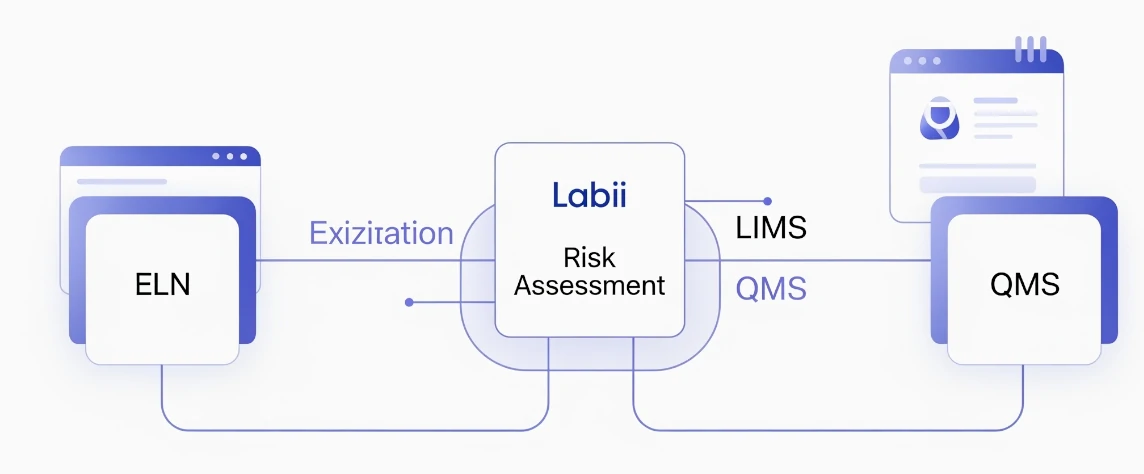
Associate risks with experiments, production runs, deviations, CAPAs, or SOPs. Labii’s interconnected platform ensures risk awareness is embedded across your operations and scientific workflows.
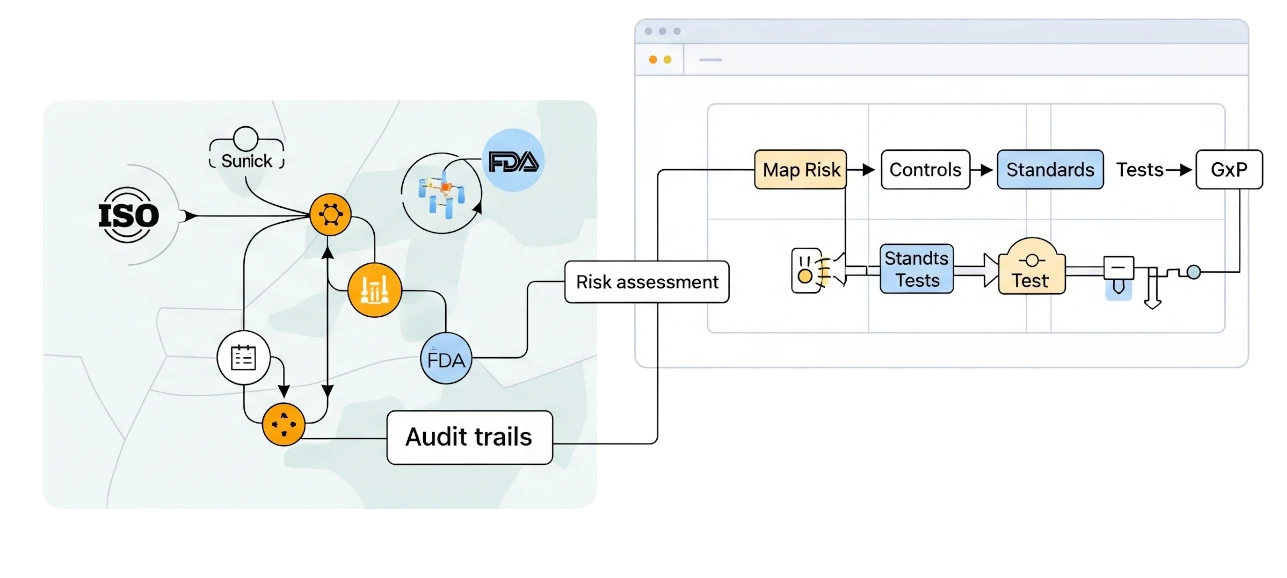
Regulatory Compliance Made Easy
Designed for regulated environments, Labii provides built-in audit trails, digital signatures, and compliance-ready reporting. Map each risk to controls, standards, and tests to satisfy ISO, FDA, and GxP requirements.
Ready to Build Your Own Scientific Applications?
See how Labii can transform your research management. Schedule a live demo or start configuring your system today. Whether you’re looking for a complete ELN/LIMS replacement or building your own lab workflows, Labii gives you everything you need to start fast and scale smart.
Refer a company to Labii and earn free months for each seat they sign.